Telangana moves to emergency footing over Coldrif cases
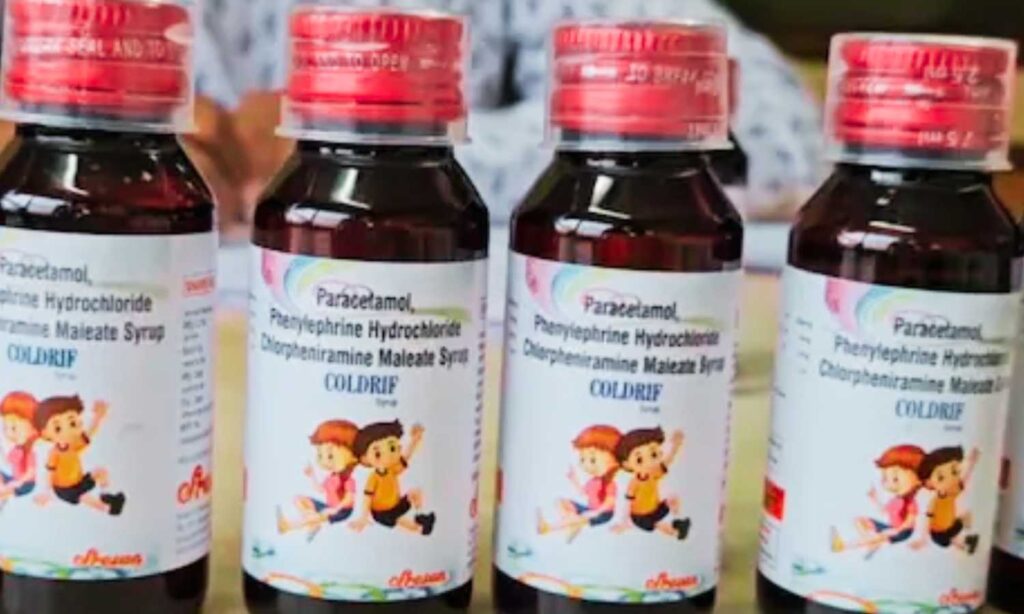
Hyderabad: Telangana has activated an emergency response after reports of about 14 child deaths in Madhya Pradesh and Rajasthan linked to Coldrif cough syrup. The Health Department has issued statewide instructions to curb exposure, trace stock, and protect infants.
Key points for the public and providers:
- Under-2 advisory: Avoid cough/cold syrups for children under two.
- First-line care: Home remedies, fluids, rest for routine symptoms.
- GMP-only: Prescribe only GMP-compliant syrups when truly needed.
- Immediate recall: Batch SR-13 (Mfg May 2025; Exp Apr 2027) suspected of diethylene glycol contamination.
- Report now: Call 1800-599-6969 and notify local Drug Control.
Coldrif cough syrup advisory for Telangana: steps, contacts, compliance
District Medical and Health Officers will lead awareness drives across hospitals, clinics, and pharmacies. Retailers must isolate SR-13 stock, stop sales, and share invoices for tracing. Surprise checks will verify compliance. Labs have been placed on standby for confirmatory testing if required.
Clinicians have been asked to watch for red-flag symptoms in infants: trouble breathing, persistent fever, vomiting, lethargy, or poor feeding. Any suspected adverse reactions after Coldrif cough syrup use should be reported through pharmacovigilance channels. Parents should not self-medicate infants and must seek prompt care if symptoms worsen.
The department is preparing multilingual notices to reach all communities. It also urged professional associations to circulate the advisory across private networks. Transparency and speed, officials said, will keep children safe while the technical review continues.
Authorities stressed that the measures are precautionary but urgent. With cooperation from parents, providers, and retailers, Telangana aims to locate the remaining SR-13 and minimise risk quickly.




